Jijie Chai, Hong-Wei Wang Groups in Tsinghua University collaborating with Jian-Min Zhou Group in Chinese Academy of Sciences published two pieces of Research Articles in Science, revealing the molecular steps in plant immune receptor activation and defining the plant resistosome
Two landmark studies from the laboratories of Professor Jijie Chai, Professor Hong-Wei Wang in Tsinghua University and Professor Jian-Min Zhou in Chinese Academy of Sciences provide unprecedented structural insight into how plant immune receptors are primed – and then activated – to provide plants with resistance against microbial pathogens, which are published as bac-to-bac Research Articles in Science on April 5, 2019. Two papers are entitled “Ligand-triggered allosteric ADP release primes a plant NLR complex” and “Reconstitution and structure of a plant NLR resistosome conferring immunity”, respectively.
Although separated by more than one billion years of evolution, plants and animals have hit upon similar immune strategies to protect themselves against pathogens. One important mechanism is defined by cytoplasmic receptors called NLRs that, in plants, recognize so-called effectors, molecules that invading microorganisms secrete into the plant’s cells. These recognition events can either involve direct recognition of effectors by NLRs or indirect recognition, in which the NLRs act as ‘guards’ that monitor additional host proteins or ‘guardees’ that are modified by effectors. Host recognition of effectors, whether direct or indirect, results in cell death to confine microbes to the site of infection. However, until now, a detailed understanding of the mechanisms of action of plant NLRs has been lacking, and much of our understanding of how these molecules function in plants has been based on comparison with animal counterparts.
In two new studies published in the journal Science, Jijie Chai and his laboratory together with the groups of Hong-Wei Zhang and Jian-Min Zhou at Tsinghua University and the Chinese Academy of Sciences in Beijing have now pieced together the sequence of molecular events that convert inactive NLR molecules into active complexes that provide disease resistance.
The authors focused their attentions on a protein called ZAR1, an ancient plant molecule that is likely to be of broad importance since it interacts with multiple ‘guardees’ to recognize unrelated bacterial effectors.
Using cryo-electron microscopy, Chai and co-authors observed that in the absence of bacterial effectors, ZAR1, together with the plant protein RKS1, is maintained in a latent state through interactions involving multiple domains of the ZAR1 protein (Figure 1A). Upon infection, a bacterial effector modifies the plant ‘guardee’ PBL2, which then activates RKS1 resulting in huge conformational changes that first allow plants to swap ADP for ATP (Figure 1B and 1C) and then result in the assembly of a pentameric, wheel-like structure that the authors term the ‘ZAR1 resistosome’ (Figure 2).
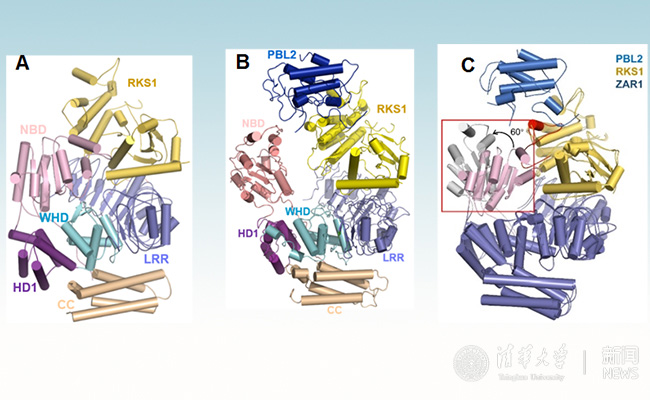
Figure 1 Overall structure of a plant NLR in inhibition form (A), priming form (B) and priming mechanism (C)
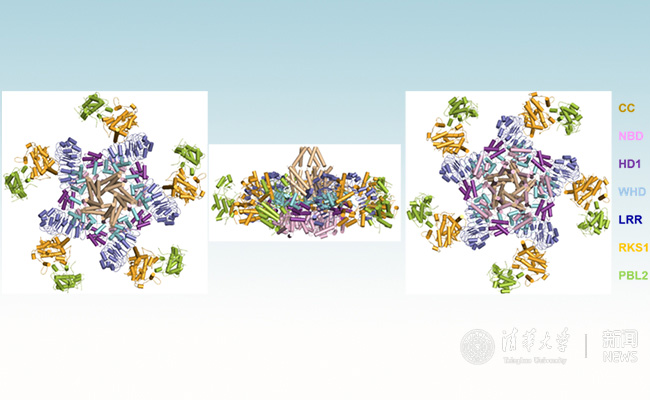
Figure 2 Overall structure of plant resistosome
One striking feature of this structure is its similarity with animal NLR proteins, which, once activated, also assemble into wheel-like structures that act as signaling platforms for cell death execution and immune signaling. However, one important difference between the structures offers a tantalizing clue as to how ZAR1 induces cell death. The authors could identify a highly ordered funnel-like structure in ZAR1 that tethers the resistosome to the plasma membrane and is required for cell death and disease resistance. The authors speculate that ZAR1 may form a pore in the plasma membrane and in this way perturb cellular function leading to immune signaling and cell death (Figure 3).
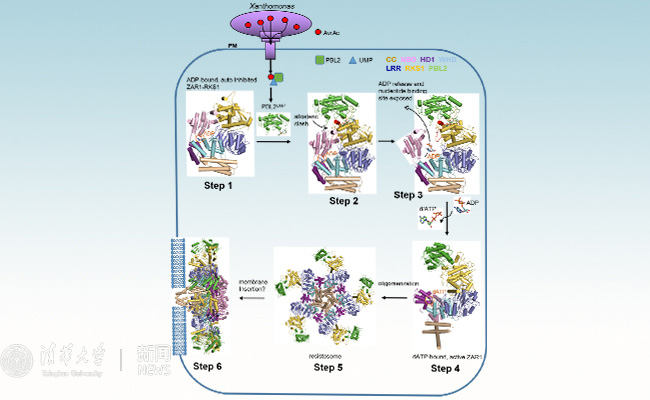
Figure 3 Molecular mechanisms of plant resistosome
Other plant NLRs also assemble into complexes that associate with the plasma membrane and it is thus highly likely that Chai’s findings have important general implications for understanding plant immunity. MPIPZ director Paul Schulze-Lefert, who was not involved in the studies, is in no doubt about the importance of the new studies: “This will become textbook knowledge.”
Prof. Jijie Chai, Hong-Wei Wang from School of Life Sciences, Tsinghua University, and Dr. Jin-Min Zhou from Institute of Genetics and Developmental Biology are the corresponding authors of the two papers; Jizong Wang (Postdoctor) , and Jia Wang (Postdoctor) from School of Life Sciences, Tsinghua University, and Meijuan Hu (graduate) from CAS Institute of Genetics and Developmental Biology are co-first authors. The Tsinghua University Branch of the China National Center for Protein Sciences (Beijing) for providing the cryo-EM facility support and the computational facility support on the cluster of Bio-Computing Platform. This research in Tsinghua University was funded by National Natural Science Foundation of China, the National Key R&D Program of China, and the Beijing Municipal Science & Technology Commission.
The paper links:
http://science.sciencemag.org/content/364/6435/eaav5868
http://science.sciencemag.org/content/364/6435/eaav5870
http://science.sciencemag.org/content/364/6435/31
(From School of Life Sciences)

