Understanding antennae-like phycobilisomes, and the complex mechanisms of photosynthesis in organisms such as red algae may be key to low-light artificial photosynthesis.
If artificial photosynthesis is ever realized, the unique light harvesting abilities of organisms that live in low light could inspire super-efficient solar conversion systems that could be stored under the vast ocean surface, says Sui Sen-Fang, a professor at Tsinghua’s School of Life Sciences.

Red alga Griffithsia pacifica
Sen-Fang is using advanced cryogenic electron tomography to study the macromolecular structures of a uniquely efficient group of light-converting organisms, including cyanobacteria and red algae.
These organisms all use photosynthesis enhanced by antenna-like structures that capture and transfer light energy known as phycobilisomes. “Most organisms using phycobilisomes are adapted to habitats with low light and blue-green light 20 meters or more below the ocean surface,” he explains. “Thus, they need the large and high-efficient phycobilisome light harvesting antenna to capture limited light energy.”
Significant structures
Phycobilisomes, Sen-Fang’s group are revealing, have an orderly efficiency. In photosynthesis, light is absorbed by chromophores and in red algae. Energy transfer is then mediated by chromophores that covalently bind to phycobilisomes composed of phycobiliproteins and linker proteins. These phycobilisomes contain hundreds to thousands of chromophores that ensure a vast absorption of light energy.
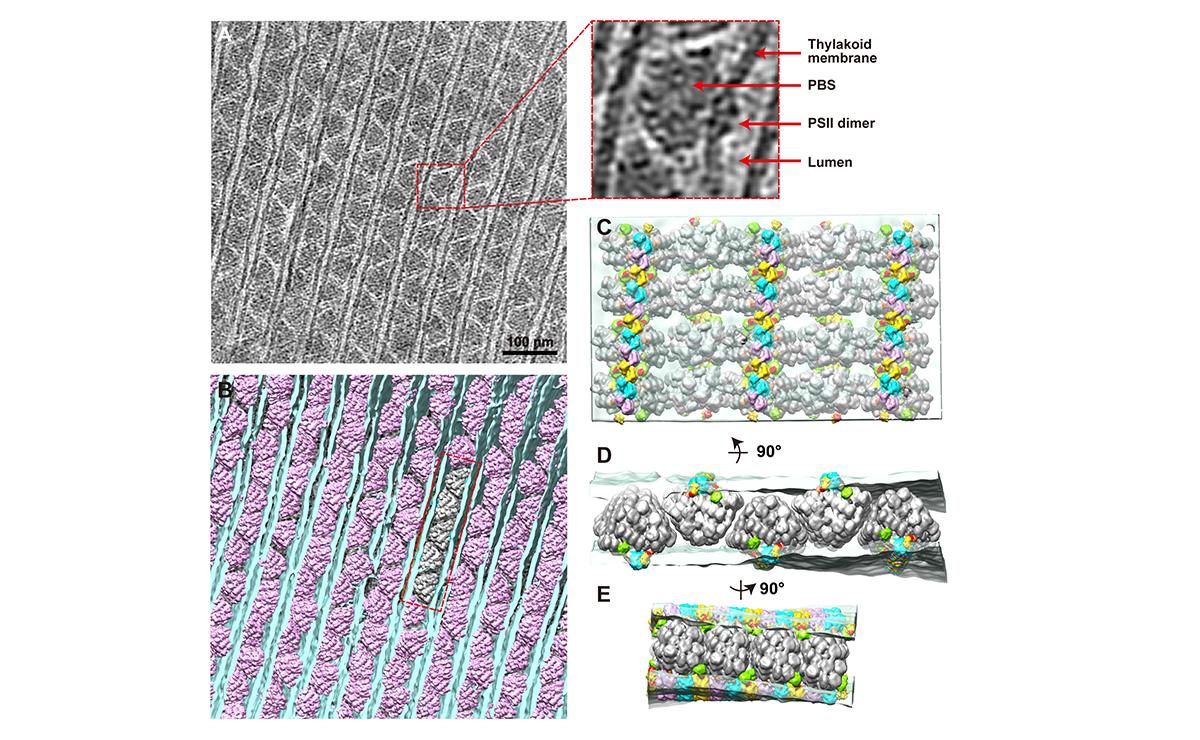
(A) A tomogram cross-section of phycobilisome–photosystem II supercomplexes. (B) Spatial mapping of phycobilisomes (purple) attached to photosystem II dimers in the thylakoid membrane (blue). (C–E) Insets from (B) at different angles. Thylakoid membrane (light blue) and phycobilisome (grey).
(© Ref 1. eLife Sciences Publications Ltd. Licenced via CC BY 3.0 https://creativecommons.org/licenses/by/3.0/)
The energy they absorb is then used by photosystem II, a pigment-protein complex, to catalyze water oxidation and release oxygen.
Recently, Sen-Fang’s group have been using in situ cryogenic electron tomography in combination with cryogenic focused ion beam milling to directly dissect key macromolecular structures involved in photosynthesis in the undisturbed cellular environment at molecular resolution. By using this approach, they are able to study native phycobilisome-photosystem II complexes by bypassing the most difficult isolation step usually needed for in vitro study.
“Phycobilisomes are water-soluble, while photosystem II is a transmembrane protein complex that needs detergent to remain soluble in vitro,” explains Sen-Fang. “We usually use detergent to purify a phycobilisome-photosystem II complex. However, detergent can affect the binding between phycobilisomes and photosystem II and lead to the disassembly of the complex, which previously made it difficult to obtain intact complexes.”
The group’s recent high-resolution cryo-electron microscopy imaging of intact phycobilisome structures has revealed that their most striking feature is a scaffold formed by the linker proteins. Phycobiliproteins are assembled on the scaffold in a well organized manner. Thus, all chromophores are also kept in order1.
Importantly, an analysis by the team published in 2020 in Nature demonstrated that the linker proteins not only control the assembly of phycobiliproteins into phycobilisomes, but are extensively involved in the modulation of the energy states of the chromophores through various dispersion forces that ensure the efficient unidirectional energy transfer2.
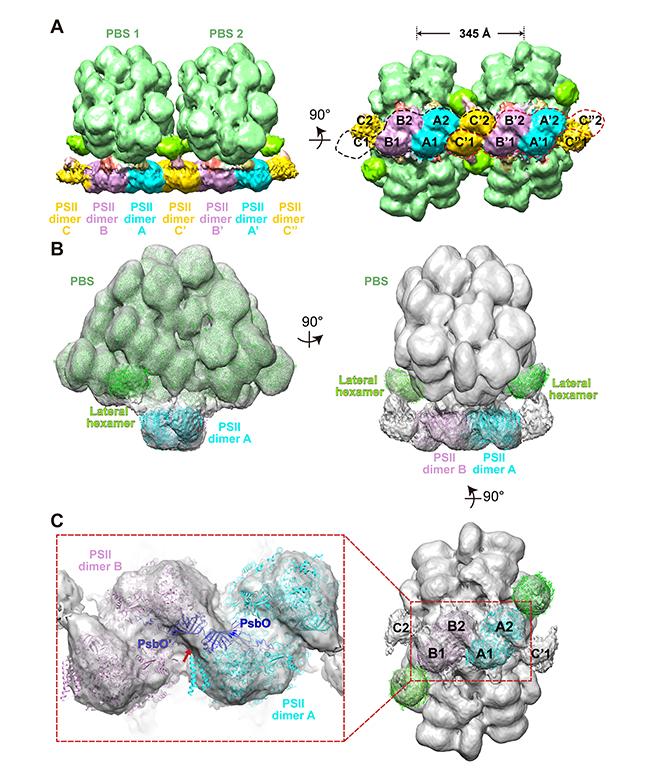
(A) The structure of a double phycobilisome–photosystem II supercomplex at a resolution of 15.6 Å. (B) The structure of a single phycobilisome–photosystem II supercomplex at a resolution of 14.3 Å. (C) Photosystem II dimers A and B, and (inset) two PsbO subunits bind with each other at the interface of adjacent photosystem II dimers.
(© Ref 1. eLife Sciences Publications Ltd. Licenced via CC BY 3.0 https://creativecommons.org/licenses/by/3.0/)
Light adjustable
The group has also looked closely at the biogenesis and repair of photosystem II. In a study published in PNAS in 2021, Sen-Fang’s group looked at binding sites of assembly factors cyanobacteria at the luminal surface of photosystem II.
They found that the binding sites of Psb27, an assembly factor that plays important roles in the repair of photosystem II, largely overlaps with that of PsbQ or PsbQ′ proteins,3. These proteins help plants to grow under ever-changing environmental conditions.
“Based on this observation, we speculated that this binding site may bind to different subunits with similar 3D structures at different stages, for instance, binding to Psb27 during the repair cycle and binding to PsbQ to maintain high activity at a more mature stage,” says Sen-Fang.
The better researchers understand these complex living systems, says Sen-Fang, the easier it is for those pursuing artificial photosynthesis, an emerging technology, inspired by natural photosynthesis, that aims to produce hydrogen or other industrially useful compounds using CO2, water, and sunlight.
Not only could highly sensitive artificial photosynthesis technology make use of the planet’s vast water-covered surface area, but it could absorb large quantities of carbon at the same time.
References
1. Li, M., Ma, J., Li, X. & Sui, S.-F. In situ cryo-ET structure of phycobilisome-photosystem II supercomplex from red alga. elife 10, (2021) doi: 10.7554/eLife.69635
2. Ma, J., You, X., Sun, S., Wang, X., Qin, S. et al. Structural basis of energy transfer in Porphyridium purpureum phycobilisome.Nature 579(7797), 146-151 (2020) doi: 10.1038/s41586-020-2020-7
3. Huang, G. Xiao, Y., Pi, X., Zhao, L., Zhu, Q. et al. Structural insights into a dimeric Psb27-photosystem II complex from a cyanobacterium Thermosynechococcus vulcanus PNAS 118, (2021) doi: 10.1073/pnas.2018053118