Recently, Group of Associate Professor Miao Li from the School of Environment have developed a kind of metal–organic framework-derived Co-doped Fe/Fe2O3 catalysts, and realized the efficient electrochemical removal of nitrate and sustainable energy recovery. The research results were published online in Proceedings of the National Academy of Sciences of the United States of America under the title of “High ammonia selective metal–organic framework-derived Co-doped Fe/Fe2O3 catalysts for electrochemical nitrate reduction”.
Nitrate (NO3-) is one of the world’s most widespread water pollutants. High concentrations of NO3- have been concerned as a serious threat to ecological balance and human health. The conversion or removal of NO3- is an important challenge that is necessary to restore the globally perturbed nitrogen cycle. Therefore, from an environmental and energetic point of view, the electrochemical conversion of NO3- to NH3 is a facile and efficient purification method and will provide a sustainable route to restore the imbalance of the global nitrogen cycle. Nitrogen nutrients or fuel from wastewater can be recovered while removing NO3- contaminants. The development of electrode materials with low cost, high activity, and selectivity is a continuous challenge and pursuit in this field. In this study, the d electrons of transition metals are prone to the formation of metal-H bond leading to the competitive HER, which affects the catalytic efficiency and NH3 selectivity. Here, we present a metal-organic framework (MOF)-derived cobalt-doped Fe@Fe2O3 (Co-Fe@Fe2O3) catalyst by tuning the electronic band structure (as shown in Figure 1). This catalyst has a nitrate removal capacity of 100.8 mg N gcat-1 h-1 and an ammonium selectivity of 99.0 ± 0.1 %, which was the highest among present reported research by far.
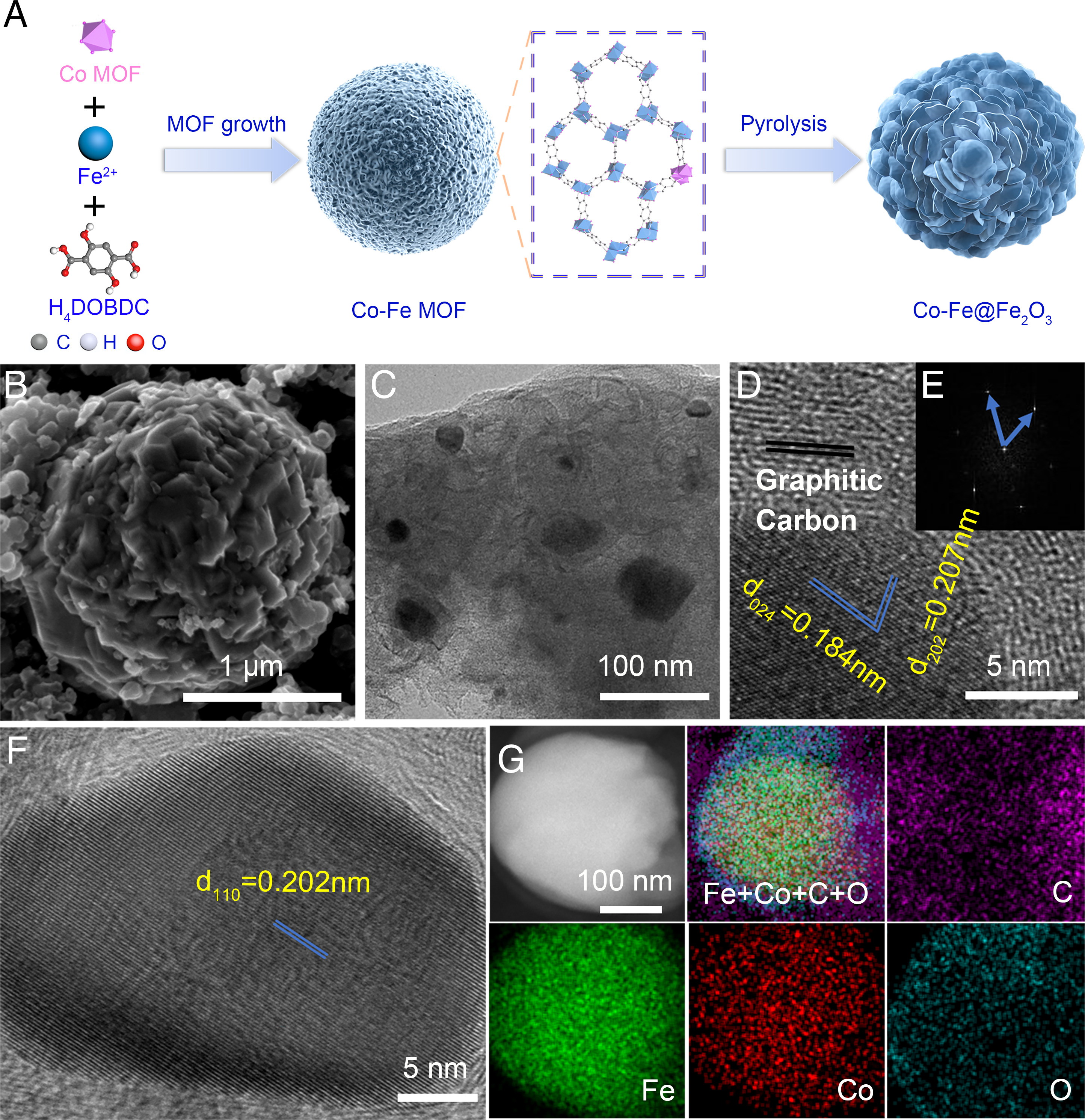
Fig. 1. Synthesis and morphology of Co-Fe@Fe2O3.
X-ray absorption fine structure spectra were obtained to reveal the structure and coordination environment of Co and Fe atoms in Co- Fe@Fe2O3. These results further support the successful formation of the desired materials. Co dopants change the electronic environment around the doping site and generate new catalytic active sites. Thus, Co doping causes a shift of the d-band centre of Fe, which changes the adsorption energy of intermediates and products, thus enhancing catalytic performance.
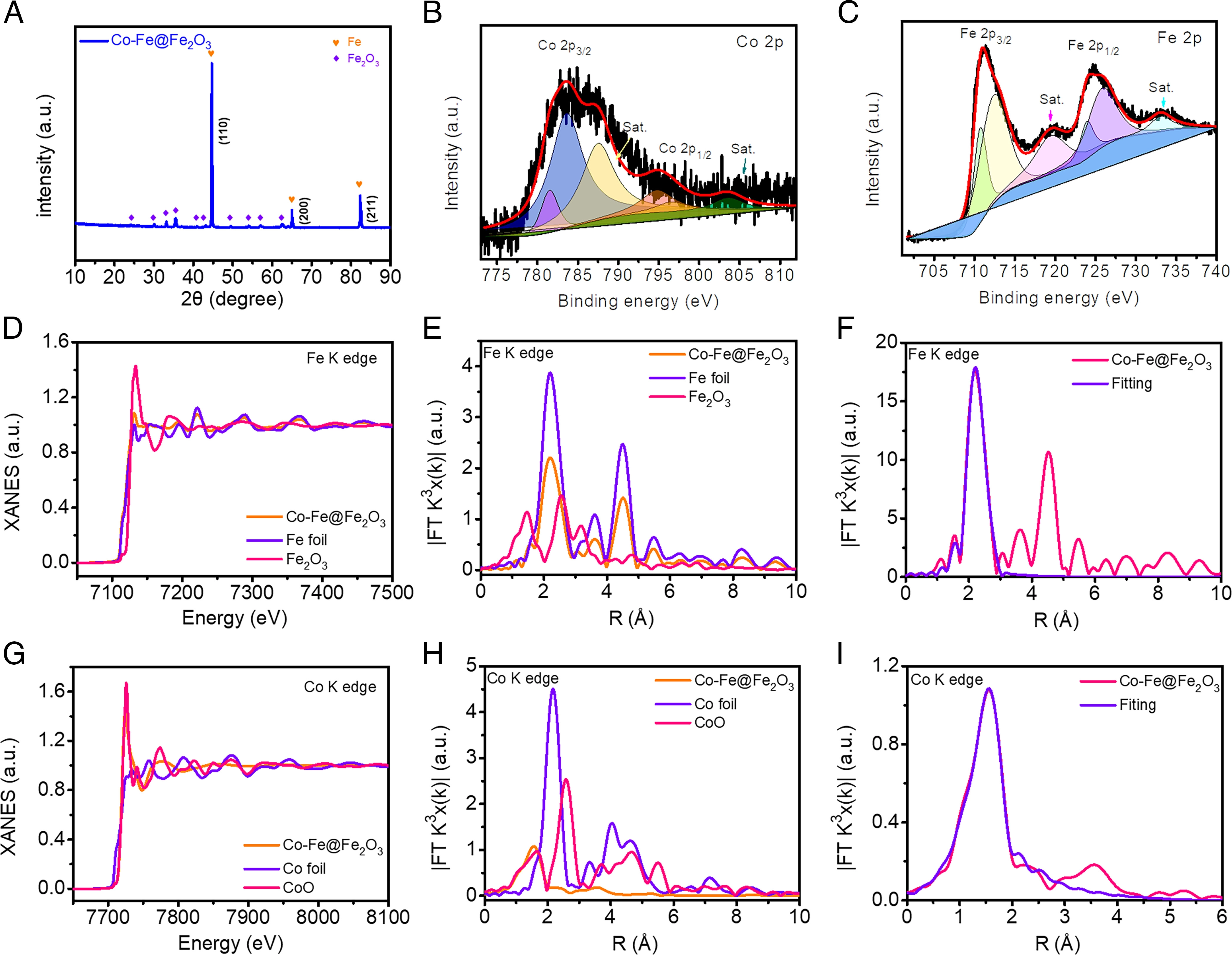
Fig. 2. Characterisation of Co-Fe@Fe2O3.
The calculations reveal the crucial role of Fe active sites neighbouring Co sites (FeCo) for NO3-reduction reaction (NO3-RR) activation. To illustrate the influence of Co doping on the reaction pathways, we calculated the free energy diagrams for NO3- reduction. These results suggest that the Co dopant activates adjacent FeCo active sites resulting in reduced energy barriers in comparison with those over pristine Fe@Fe2O3. Thus, Co and FeCo synergistically enhance the NO3-RR activity of Co-Fe@Fe2O3. To gain insight into the different NO3-RR activities of the catalysts, the d-band centres of the metal active sites were calculated. Further, with Co doping, the d-band centres of the Fe active site shifts to higher energy , whereas the ɛd of the Co dopants shifts to lower energy; thus, the binding strength of NO3- on Co-Fe@Fe2O3 is slightly weaker than that on Fe@Fe2O3. Thus, the main effects of Co doping are (1) being the key NO3-RR active sites and (2) changing the Fe 3d orbital configuration and being an activator of Fe sites, thus enhancing the intrinsic NO3-RR activity of Fe@Fe2O3. Thus, this study provides a strategy in the design and popularization of MOF-derived catalysts in other fields.
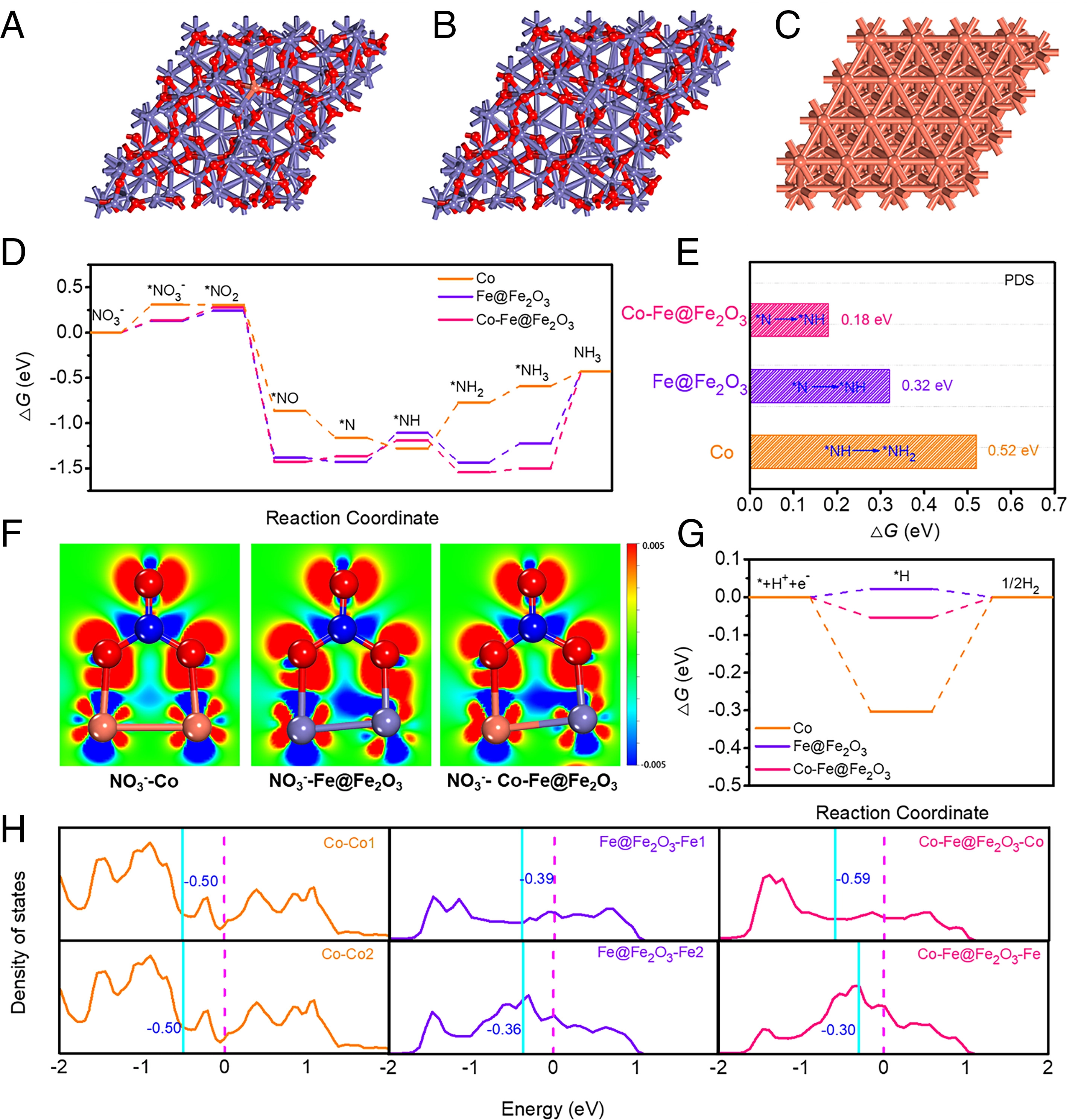
Fig. 3. Mechanistic study of catalytically active sites in Co-Fe@Fe2O3 for NO3-RR.
The first author of the paper is Shuo Zhang, a doctoral candidate of the school of environment in Tsinghua University. The corresponding author of the paper is Miao Li, an associate professor of the school of environment in Tsinghua University. The Professor Xiang Liu have provided important guidance and help for the experimental research and analysis. The research project is supported by project of the National Natural Science Foundation of China.
Paper link: https://doi.org/10.1073/pnas.2115504119